OSN-Assisted Catalyst Recycling for Cleaner Pharma
Catalysts are the silent drivers of countless chemical transformation, enabling accelerated and more efficient chemical reactions without being consumed in the process. By lowering the activation energy–the minimum energy required for a reaction to occur, they make it possible to achieve higher reaction rates, improved yields, and better energy efficiency. This fundamental ability makes them indispensable across a range of industries, including pharmaceutical, petrochemical, fine chemicals, and more. Additionally, catalysts frequently offer alternative reaction pathways, which further enhance selectivity and efficiency.
Catalysis is generally divided into two main categories: homogenous and heterogeneous. In homogenous catalysis, the catalyst and reactants exist in the same phase, typically a solution. In heterogenous catalysis, the catalyst is in a different phase–most commonly a solid interacting with liquid or gas-phase reactants. In general, over 90% of all chemical products involve at least one catalytic step, and while both homogenous and heterogenous are widely used, heterogenous catalysis is particularly dominant in industrial settings due to its practical advantages, including greater robustness, ease of separation from reaction mixtures, and lower operational costs. The ability to reuse solid catalysts simplifies downstream processing, reduces waste, and supports more streamlined, scalable operations. Therefore, heterogenous catalysis is a cornerstone of modern chemical manufacturing. However, despite its advantages, heterogenous catalysis also presents a set of technical challenges that can impact the efficiency, cost-effectiveness, and sustainability of particular processes. For instance, catalyst deactivation is a challenge that is caused by chemical impurities or by-products that accumulate on the catalyst’s surface and block active sites. This fouling reduces the catalyst’s activity and selectivity, necessitating frequent regeneration or replacement–both of which increase operational complexity and cost. Thus, addressing these limitations is critical for maintaining process efficiency and long-term economic viability.
In contrast to heterogenous systems, homogenous catalysts offer several significant advantages, including high selectivity for target products, exceptional catalytic activity at mild temperatures and pressures, and mechanisms that are more easily studied due to the catalyst’s solubility. These characteristics make homogenous catalysis particularly valuable in many industries where precision and control are paramount. However, the recovery and reuse of homogenous catalysts remain a major barrier. Traditional separation techniques, such as distillation, are often energy-intensive and technically challenging, especially in complex reaction systems. For instance, in the manufacturing process of active pharmaceutical ingredients (APIs), distillation alone can account for as much as 70% of the total production costs, highlighting the need for more energy-efficient and economically viable alternatives.
Homogeneous Catalyst Recycling: A Step-by-Step Breakdown
Homogenous catalyst recycling is a technically demanding process. The following breakdown outlines some of the key steps involved:
1. Reaction Completion
After the catalytic reaction concludes, the mixture is typically quenched to stop further activity and stabilize the catalyst. This step is critical to prevent further degradation and unwanted side reactions.
2. Separation of the Catalyst
As homogenous catalysts exist in the same phase as the reactants, their separation requires advanced techniques. Some common and well-established techniques include:
Distillation: An effective method when the catalyst and products have varying boiling points.
Liquid-Liquid Extraction: This technique is effective if the catalyst and products are soluble in different solvents.
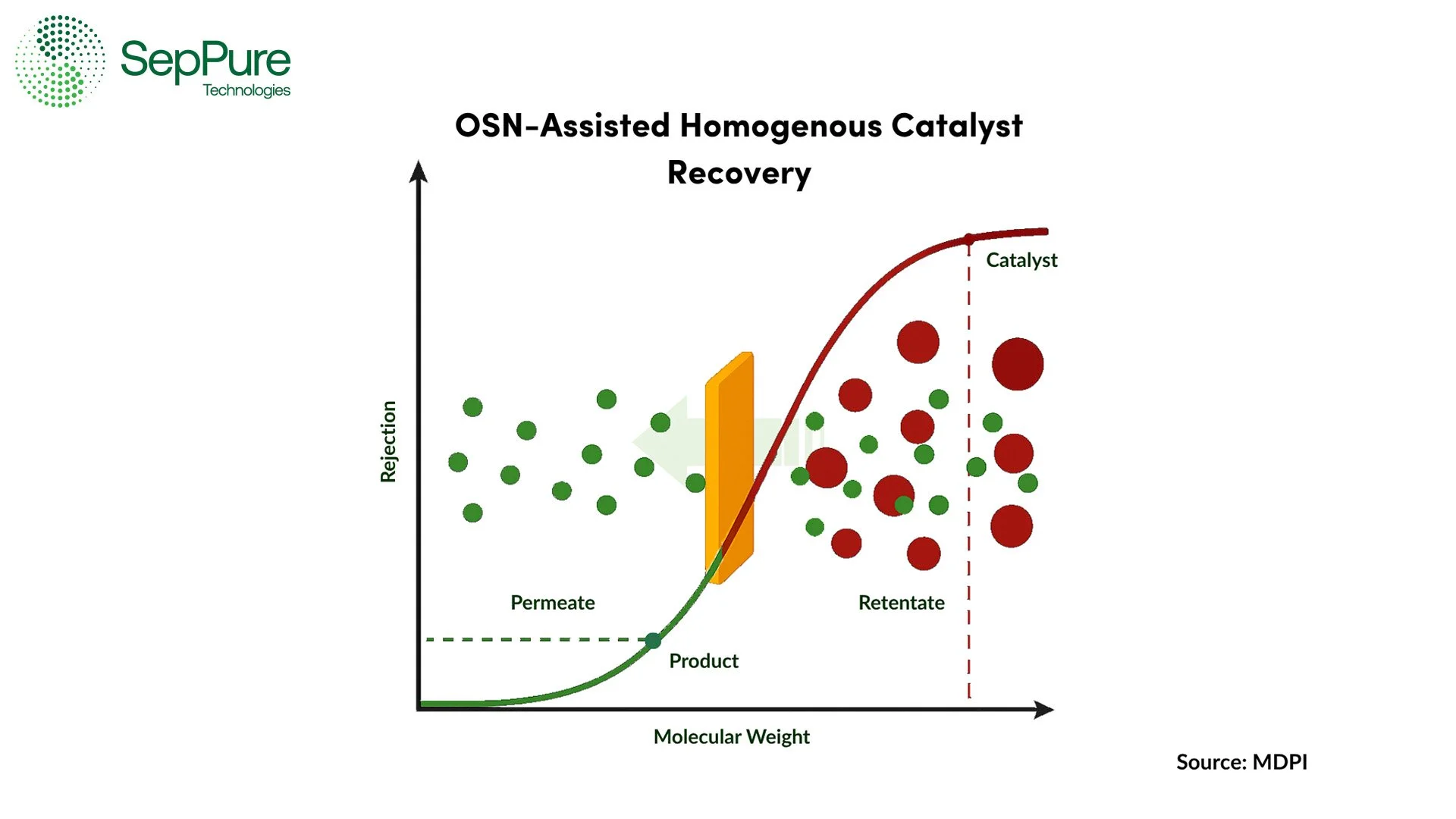
Nanofiltration: Involves Organic Solvent Nanofiltration (OSN) membrane technology to separate molecules based on their molecular weight and charge, enabling selective recovery of the catalyst.
And further particular techniques.
3. Regeneration of the Catalyst
Over time, homogeneous catalysts may degrade or lose effectiveness. Regeneration is a key step in the recycling process. This involves applying specific conditions (e.g., temperature, solvents, or other chemical agents) to restore the catalyst's activity. Techniques like oxidation-reduction or chemical treatment can help regenerate catalysts, returning them to a usable state for subsequent reactions.
4. Reuse of the Catalyst
Once regenerated, the catalyst can be reused in new reaction cycles. Not only reduces reliance on fresh raw materials, but also helps cut operational costs and waste, supporting environmental sustainability and compliance with waste management regulations.
Implementing an effective homogenous catalyst recycling process offers numerous performance, environmental, and economic advantages. It enables the precise recovery of homogenous catalysts, promotes sustainability, and enhances process efficiency across industries.
Homogeneous Catalyst Recycling; Current Methods & Challenges
Homogeneous catalyst recycling refers to the recovery and reuse of catalysts that are in the same phase as the reactants within a chemical reaction. These catalysts are commonly organometallic complexes or soluble acids and bases, and they are widely used in fine chemicals, petrochemicals, and pharmaceutical production due to their distinct advantages, including high catalytic activity, excellent selectivity, and well-defined active sites that enable precise control over reaction pathways. However, despite their benefits, homogeneous catalysts pose significant challenges when it comes to separation and reuse. Because they are in the same phase as the reactants, they cannot be easily removed through simple physical means like filtration, unlike heterogeneous catalysts, substances that exist in a different phase. A major category of homogeneous catalysts includes complexes of transition metals, especially those involving platinum group metals such as palladium, rhodium, and ruthenium. These metals are not only costly but also tightly regulated due to their toxicity and potential contamination of final products, particularly in pharmaceutical and food-related applications. Therefore, their efficient recovery is not just economically advantageous, but often a regulatory necessity. To address this, various separation and recycling methods are employed. One of the most established techniques is distillation, which involves vaporizing the reaction mixture and selectively condensing its components based on their boiling points. This method can be effective for separating volatile products from non-volatile catalysts; however, it still encounters several limitations:
Energy Intensity: Distillation is highly energy-intensive and contributes to significant energy consumption and greenhouse gas (GHG) emissions.
High Expenses: Distillation is costly, leading to high overall operational costs. The use of distillation already accounts for 70% of production costs.
Thermal Sensitivity: Many organometallic catalysts degrade at high temperatures, making them incompatible with traditional distillation processes.
Incomplete Separation: In some cases, azeotropes or overlapping boiling points prevent clean separation, leading to catalyst loss or contamination.
Because of these drawbacks, there is growing interest in more efficient and sustainable alternatives for homogeneous catalyst recycling, such as membrane-based separations, biphasic systems, or catalyst immobilization techniques that retain homogeneous behavior while enabling easier recovery.
OSN Membrane-Assisted Homogeneous Catalyst Recycling
While conventional separation methods including distillation, extraction and chromatography are well-established and widely applied across various sectors, they present significant challenges in catalyst recycling, particularly in systems employing homogenous catalysts. As previously demonstrated, in contrast to heterogenous catalysts, homogenous catalysts are molecularly dispersed in the reaction medium. Therefore, conventional separation methods are not often the most efficient. For instance, distillation may not be feasible when the catalyst and reactants have closer boiling points or when the catalyst is thermally unstable. Similarly, solvent extraction can lead to the loss of valuable catalysts to waste streams, while chromatography often involves costly regenerations, has limited scalability, and leads to significant solvent consumption. These limitations not only reduce catalyst recovery rates, but they also introduce substantial operational costs and environmental burdens. This is where Organic Solvent Nanofiltration (OSN) membrane technology emerges as a transformative alternative.
Unlike traditional thermal or solvent-intensive techniques, Organic Solvent Nanofiltration (OSN) membrane technology enables the separation of chemicals based on their molecular properties, such as size and charge, under mild operating conditions, typically involving only a pressure gradient. Since no phase change is required, energy consumption is dramatically reduced, and the chemical integrity of both products and catalysts is better preserved. These membranes can selectively retain larger homogenous catalysts while allowing smaller product molecules and solvents to pass through and vice versa, facilitating an efficient and energy-saving route to catalyst recycling. Some of the key advantages of utilizing OSN membranes are as follows:
1. High Selectivity
Organic Solvent Nanofiltration (OSN) membrane technology enables separation based on molecular weight. This is especially beneficial when the catalyst and product are similar in polarity or solubility, conditions where traditional methods fail.
2. Lower Energy Requirements
Unlike distillation or evaporation, membrane separation operates under mild temperatures and does not require a phase change, making it significantly less energy-intensive. This reduces operational costs and lowers the environmental footprint.
3. Preservation of Catalyst Activity
Gentle operating conditions protect heat-sensitive catalysts from degradation, enabling their repeated use without loss of activity or selectivity, an important consideration in fine chemical and pharmaceutical processes.
4. Scalability and Integration
Membrane units are modular and can be easily scaled. They can also be integrated into continuous flow processes or hybrid systems with minimal redesign, improving process intensification and productivity.
5. Reduction in Solvent Use and Waste
By eliminating or minimizing the need for solvent extraction or chromatography, membrane processes reduce solvent consumption and generation of hazardous waste. This aligns well with green chemistry and sustainability goals.
6. Compatibility with a Wide Range of Solvents
SepPure’s Organic Solvent Nanofiltration (OSN) membrane technology is the only nanofiltration technology in the market that is chemically resistant to both polar and polar-aprotic solvents, enabling their use in diverse chemical systems.
Conclusion
Recycling homogeneous catalysts remains a significant challenge in chemical manufacturing for numerous reasons. Their homogeneity complicates their separation and recovery using conventional methods. Techniques such as distillation are highly energy-intensive and largely ineffective for separating catalysts from reaction mixtures, while chromatographic methods are costly and difficult to scale for continuous or large-scale industrial operations. However, Organic Solvent Nanofiltration (OSN) membrane technology presents a transformative solution to these challenges. This technology utilizes semi-permeable membranes to achieve selective separation based on molecular size and charge, enabling the efficient retention and recovery of homogeneous catalysts directly from chemical mixtures without the need for a phase change. This pressure-driven process not only minimizes operational costs, but also reduces solvent and catalyst loss, thereby improving overall process sustainability. By offering a scalable, energy-efficient, and environmentally friendly alternative, OSN membrane technology has emerged as one of the most promising technologies for the recovery and reuse of homogeneous catalysts. Its adoption supports both economic efficiency and the broader goals of green chemistry and sustainable manufacturing.