Advanced Nanofiltration Solutions for Sustainable APIs
Active pharmaceutical ingredients (APIs) are the backbone of modern medicine and one of the two fundamental components of any drug. APIs are the biologically active substances within a drug formulation responsible for producing the intended therapeutic effect. The other component, known as excipients, is the chemically inactive substance that helps deliver, stabilize, or enhance the effect of the API. In the pharmaceutical industry, APIs are far more than just molecules, they are critical elements that determine a medicine’s quality, safety, and efficacy. Because they directly influence a drug’s performance, maintaining their precision, purity, and quality throughout the API production process is vital. Every drug, from common painkillers to complex biologics, depends on the purity and potency of its APIs to achieve the desired therapeutic outcome. Hence, the API purification process is a critical step in the drug development and manufacturing process, ensuring optimal performance and compliance with strict regulatory standards.
Types of Active Pharmaceutical Ingredients (APIs)
To fully understand what an API is in the pharmaceutical industry, let’s take a closer look at its classifications.
Natural APIs
Derived from plants, microorganisms, and other natural sources, natural APIs are typically isolated through extraction processes. Due to their biological origin, these APIs are often chemically complex and highly therapeutic.
Synthetic APIs
Manufactured chemically in laboratories, synthetic APIs are designed with high precision and consistency. They are generally precise in chemical composition and often more cost-effective compared to natural APIs.
Within the above categories, APIs can be further classified into various types. For instance:
| Type of API | Description | Therapeutic Applications | Classification |
|---|---|---|---|
| Peptide APIs | Chains of amino acids that can mimic natural peptides in the body. | Diabetes, cancer, cardiovascular diseases; generally considered safe due to natural metabolism. | Biologic or synthetic. |
| Small Molecule APIs | Low molecular weight compounds; the backbone of pharmaceutical therapies. | Oral medications for infections, hypertension, pain management, and many chronic conditions. | Usually synthetic. |
| Oligonucleotide APIs | Short DNA or RNA molecules with therapeutic potential. | Neuromuscular diseases, ophthalmologic disorders, and emerging treatments for rare diseases. | Usually synthetic. |
And many more!
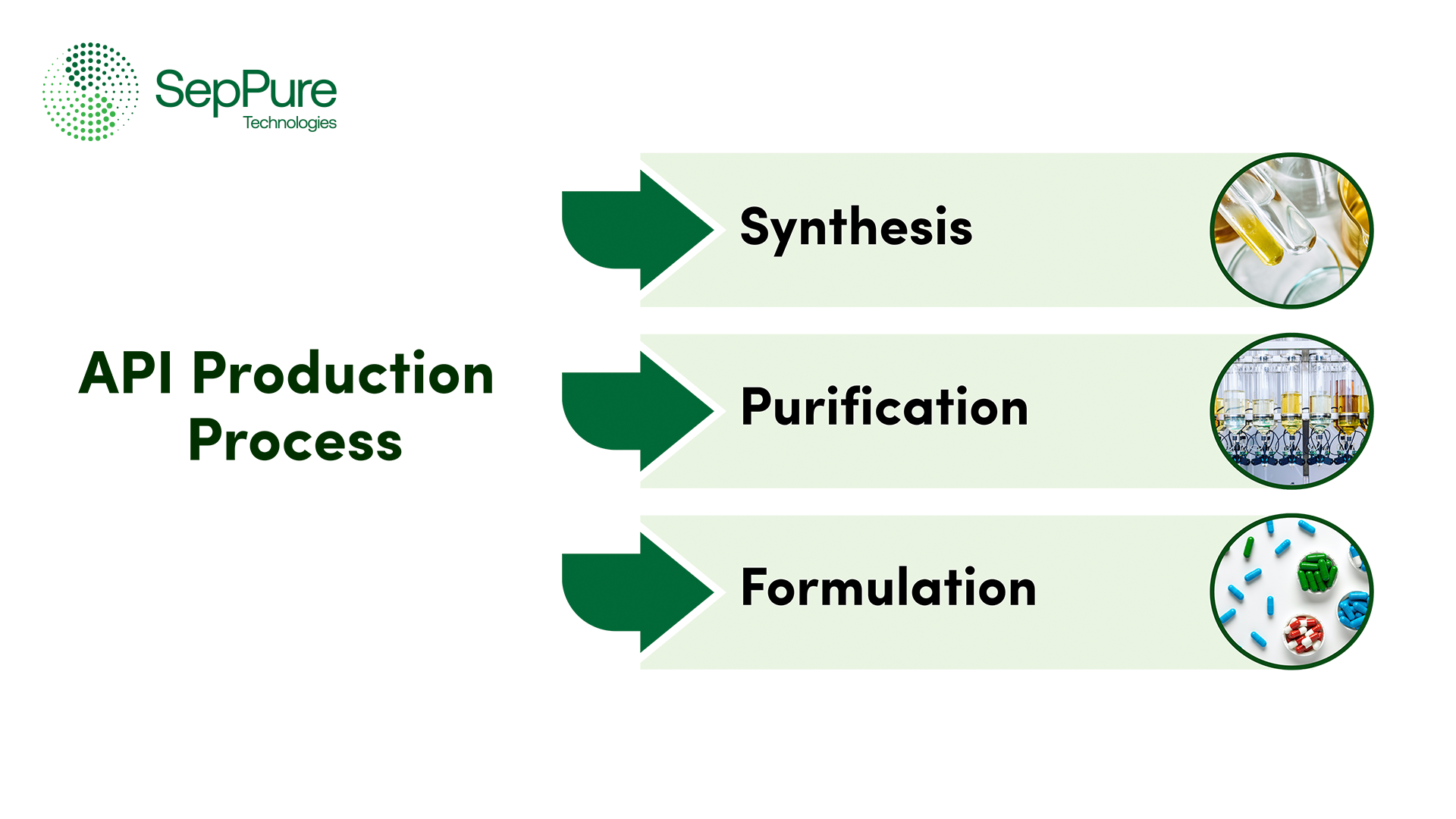
Understanding the API Production Process
The API production process involves multiple stages including synthesis, purification, and formulation.
Synthesis
First, the API is synthesized, a process that involves chemical reactions to produce the active compound. This stage can include multiple steps, including combining of the raw materials, using catalysts, and controlling the reaction conditions to produce the desired molecule. Depending on the specific chemical reactions required, synthesis can also be carried out in different mediums, involving different solvents.
Purification
After synthesis, the API product often contains impurities, including by-products or the remnants of the starting materials and solvents. The API purification phase is therefore essential to remove these impurities and ensure that the product meets the required standards. Commonly used purification techniques include crystallization, distillation, and chromatography. The purified API also undergoes drying and milling to obtain the desired particle size and consistency.
Formulation
In this stage, the API is formulated with excipients into dosage forms, including tablets, capsules, or injections. This stage ensures that the final product complies with regulatory standards for safety, efficacy, and stability, delivering reliable and effective treatment to patients.
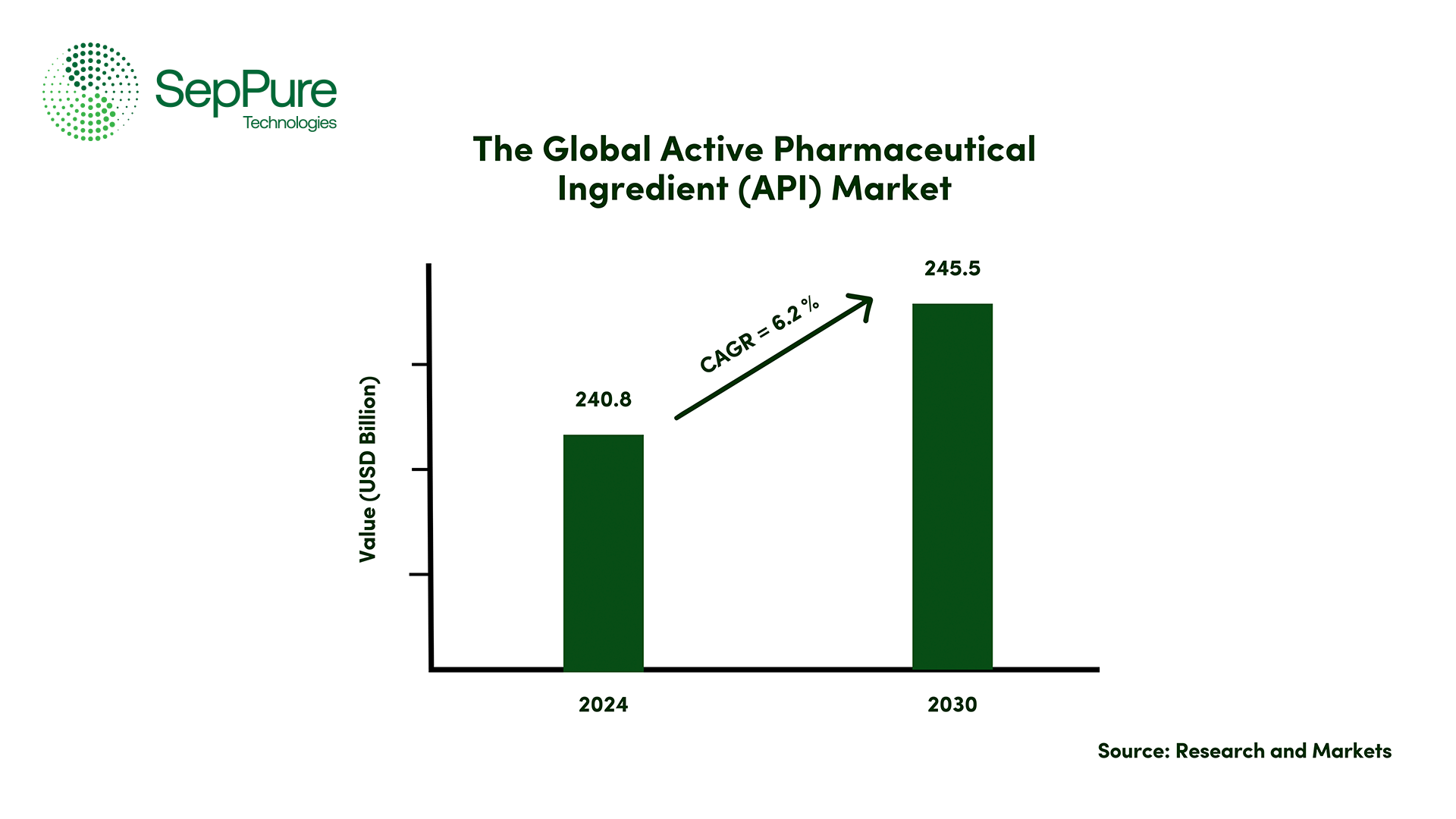
API Market Outlook & Projection
APIs or Active Pharmaceutical Ingredients represent a cornerstone of the global pharmaceutical industry. In 2024, the API market was valued at USD $240.8 billion, and it is projected to reach USD $245.5 billion by 2030, growing at a compound annual growth rate (CAGR) of 6.2%. This steady rise underscores not only the scale of the industry but also the escalating importance of APIs in addressing the growing demand for advanced therapies and innovative drug formulations. However, as the market continues to expand, the pharmaceutical industry must address many challenges to make the API production process greener, safer, and more cost-effective.
Common Challenges in API Purification
With the complexity of the purification process as well as the strict quality control, the pharmaceutical industry faces several challenges.
1. Purification Selectivity and Yield
During the purification process, the desired API is isolated from a complex mixture while ensuring that no valuable components are lost. This, however, becomes particularly challenging when dealing with chemically complex APIs or when the impurities have similar properties to the target API.
1. Solvent Exchange
As the API progresses from synthesis to purification, solvent exchange is often necessary to replace reaction solvents with those suitable for crystallization or concentrate solutions. While distillation is a standard method for solvent exchange, it can lead to the thermal degradation of the API or loss of the active ingredient due to high temperatures and prolonged exposure.
3. Solvent Waste
The API purification process requires large volumes of solvents, such as methanol, ethanol, and isopropanol. This results in substantial waste that requires transportation to specialized treatment facilities, incurring further costs. Furthermore, some solvents can be hazardous. Thus, improper disposal of these materials will not only degrade the environment but also pose a health risk.
4. Energy Consumption
Conventional separation and purification methods, such as distillation, require large quantities of heat and, by extension, vast amounts of energy. This energy-intensive process increases operational costs and contributes to higher carbon emissions, making it less environmentally friendly.
Revolutionizing API Purification with SepPure’s Advanced OSN Membranes
Traditional API production processes bring about significant challenges, including high energy consumption, extensive solvent use, and potential degradation of sensitive molecules during purification. Advanced technologies, such as SepPure’s GreenMem® Organic Solvent Nanofiltration (OSN) membranes provide a more efficient and sustainable alternative. Our membranes can be seamlessly integrated at multiple stages of the API production process. These pressure-driven membranes operate without extensive heating, achieving the same level of purity while consuming only 10% of the energy required by conventional distillation.
Controlled and Stable Solvent Exchange
Our GreenMem® Organic Solvent Nanofiltration (OSN) membranes provide a milder and more controlled separation process. By eliminating exposure to high temperatures, APIs maintain stability and retain their therapeutic properties throughout the process.
Selective Purification and Enhanced Yield
OSN membranes enable precise molecular separation based on the chemicals' size and charge. By filtering the solvent-API mixture through OSN membranes at the initial stage of downstream purification, contaminants can be efficiently removed. This ensures a higher API concentration before other purification steps, ultimately leading to improved yield and enhanced purity.
Energy-Efficient Separation
Pressure-driven separation with OSN membranes offers a substantial reduction in energy requirements compared to conventional distillation. Internal studies at SepPure Technologies demonstrate that hybrid OSN systems achieve high solvent purity while using only a fraction of the energy used by distillation.
Reduced Solvent Waste
Our GreenMem® OSN membranes enable energy-efficient recovery of solvents and catalysts, minimizing chemical waste. This cuts waste and operational costs, contributing to a circular economy in the pharmaceutical industry.
By integrating GreenMem® Organic Solvent Nanofiltration (OSN) membranes into the pharmaceutical manufacturing processes, businesses can enhance product quality, improve yield, and reduce environmental impact. This innovative technology not only ensures compliance with industry standards but also lowers operational costs and supports sustainable practices.
Frequently Asked Questions (FAQs) About APIs in Pharmaceutical Industry
-
An API or active pharmaceutical ingredient, is the biologically active component in a drug responsible for its therapeutic effect. In the pharmaceutical industry, APIs are central to a medication’s efficacy, safety, and consistency. Thus, their precise production and purification are essential.
-
API production generally involves three core stages: synthesis, purification, and formulation. Synthesis produces the active compound, purification removes impurities while preserving stability, and formulation incorporates the API into the final pharmaceutical dosage form, such as tablets, capsules, or injections.
-
API purification presents a number of challenges for the pharmaceutical industry, including solvent handling, separation selectivity, yield optimization, energy consumption, waste management, and more, all of which can affect the purity, yield, and cost-effectiveness of APIs.
-
Organic Solvent Nanofiltration (OSN) membranes offer a pressure-driven, energy-efficient approach to chemical separation processes. OSN membranes enable precise filtration, reduce energy use, minimize solvent waste, and preserve API stability, providing a sustainable alternative to traditional distillation-based methods.
-
SepPure’s GreenMem ® ️ is the world’s best solvent-resistant membrane and the only Organic Solvent Nanofiltration (OSN) technology available today that delivers high performance in both polar and polar-aprotic solvents, making it an exceptionally versatile solution for the pharmaceutical industry. By integrating GreenMem®️, the pharmaceutical industry can significantly optimize manufacturing processes, reduce waste, and energy consumption.